Diffusive-thermal instability of non-premixed turbular flame
- 1D tubular flame
- No diffusive-thermal instability
- S-curve can be obtained
- Hydrogen tubular flame (experiments)
- Diffusive-thermal instability occurs
- Lewis number of hydrogen is much less than unity
- 2D model of tubular flame
- Different number of flame cells can be observed with fuel Lewis number = 0.3
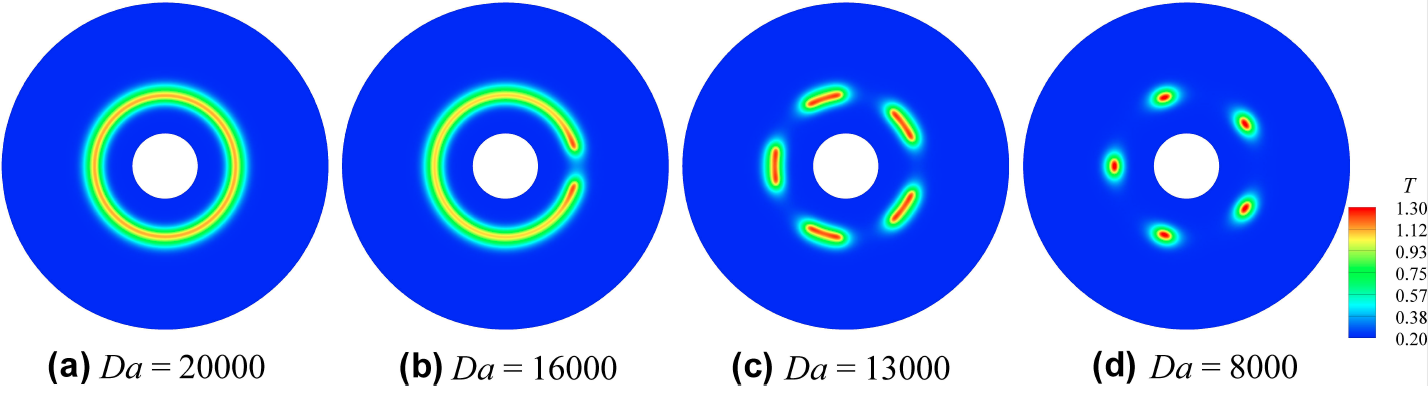
- For large Da, one tubular flame is observed
- Below a critical Da, seven, ten and twelve flame cells are observed depending on kmax from the linear stability analysis
- The number of flame cells depends upon Da, fuel Lewis number, and flame radius
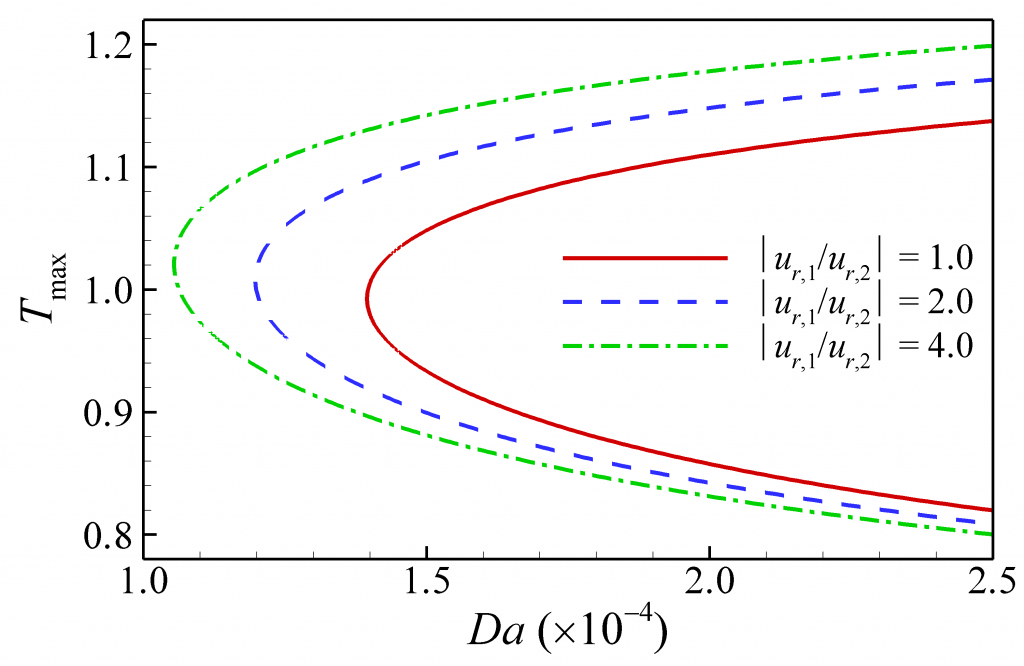
Maximum temperature vs. Damkohler number, Da in 1D tubular flame
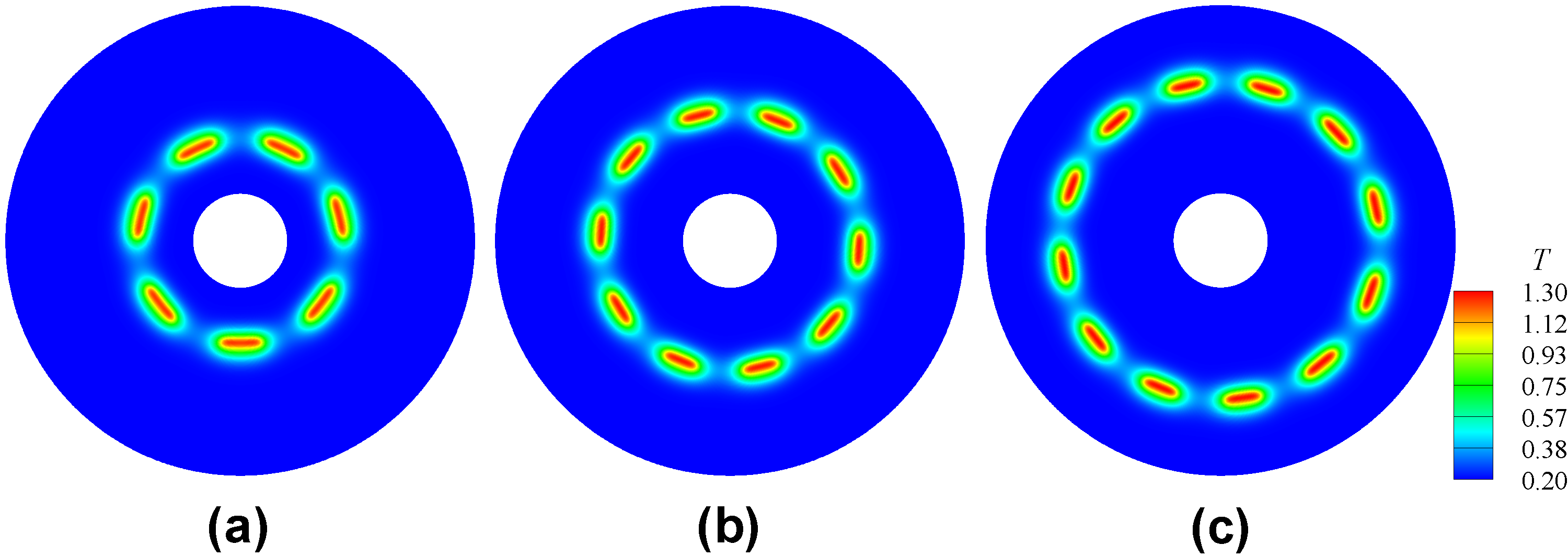
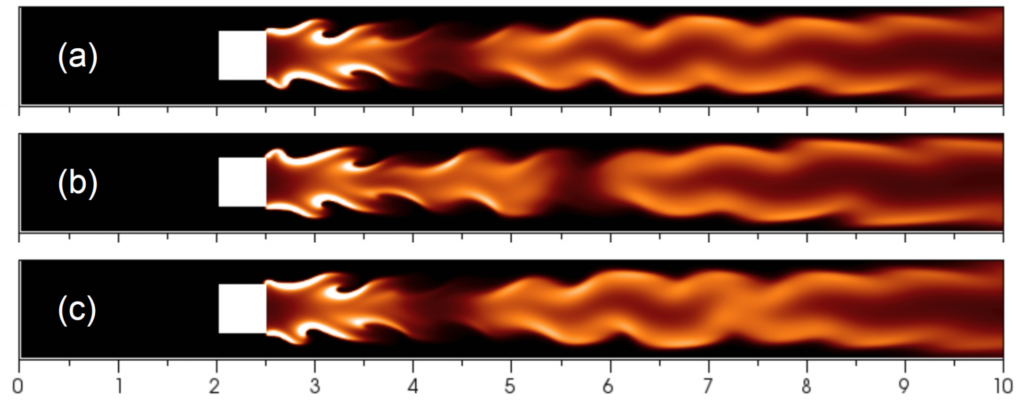
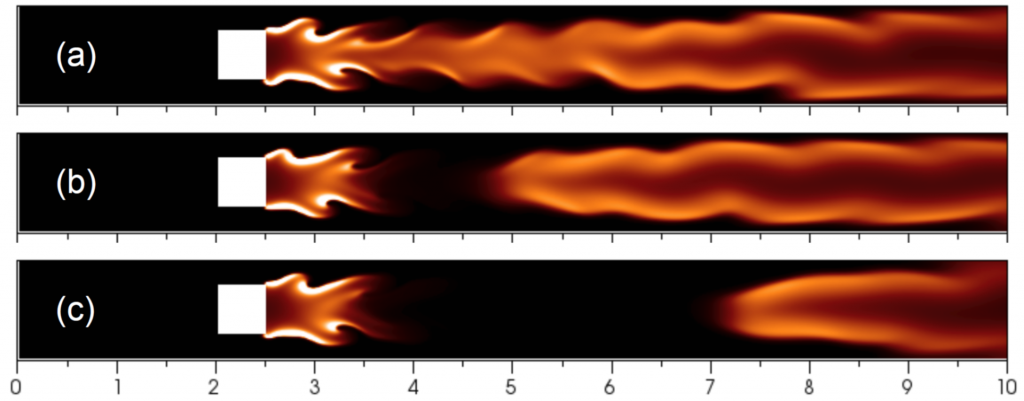
Temperature isocontours of steady flame cells with perturbed initial condition for different flame radii at their DaE=(a)13950, (b)11977, and (c)10542, respectively
Temperature isocontours of steady flame cells with C-shaped initial condition
Dynamics of a hydrogen/air premixed flame behind a bluff-body in a mesoscale combustor
Hydrogen/air premixed flame stabilization over a square cylinder in a mesoscale combustor. The vortex-shedding behind the cylinder affects the stabilization of the flames
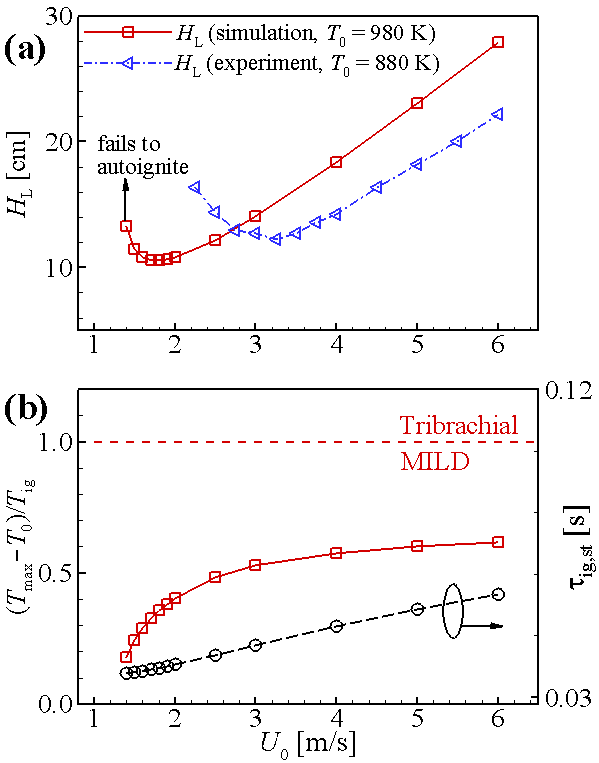
Laminar lifted jet flame under autoignitive condition
What is the lifted jet flame?
- At relatively high jet velocity, jet flames are detached from the jet nozzle and are stabilized by “lifted” status.
- Lifted flames are observed in many practical combustion appliances (e.g., Diesel engine, gas turbine)
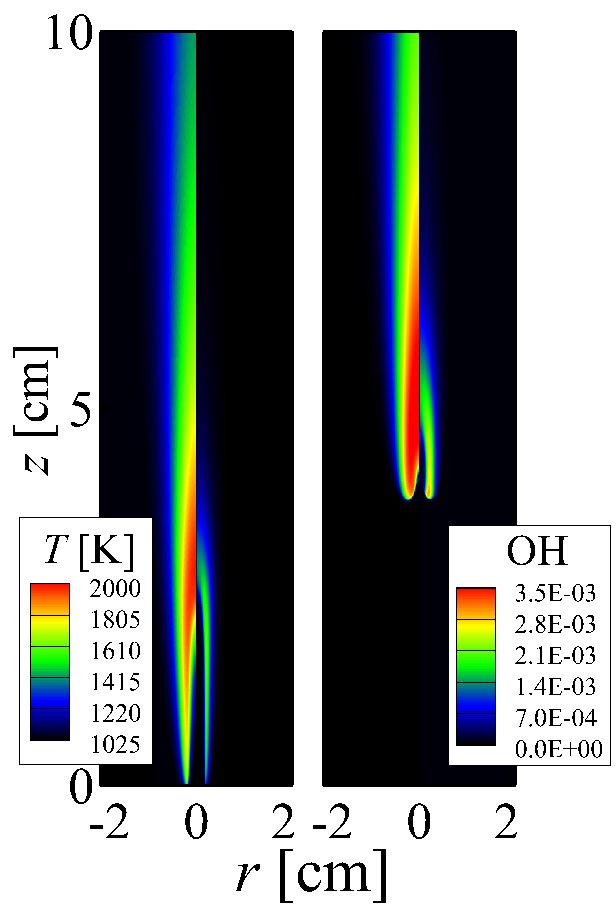
Isocontours of temperature and mass fraction of OH for autoignited laminar attached jet flame (left) and lifted jet flame (right)
Stabilization of autoignited laminar lifted jet flames
- Blowoff of lifted flames is detrimental to the combustion devices
- Liftoff height of lifted flames are highly related to the pollutant emissions
⇒ Requires a fundamental understanding of stabilization mechanism for autoignited laminar lifted flames
Autoignited Methane/hydrogen jet flame
- to elucidate the decreasing liftoff height behavior with the increasing jet velocity
- to elucidate the detailed flame structure for Tribrachial edge flame / Moderate or Intense Low oxygen Dilution (MILD) combustion
-
New Findings:
- high diffusive nature of hydrogen molecule mainly leads to the unusual liftoff height trend
- flame stabilization mechanism (autoignition, autoignition-assisted flame propagation) changes depending on the flame structures
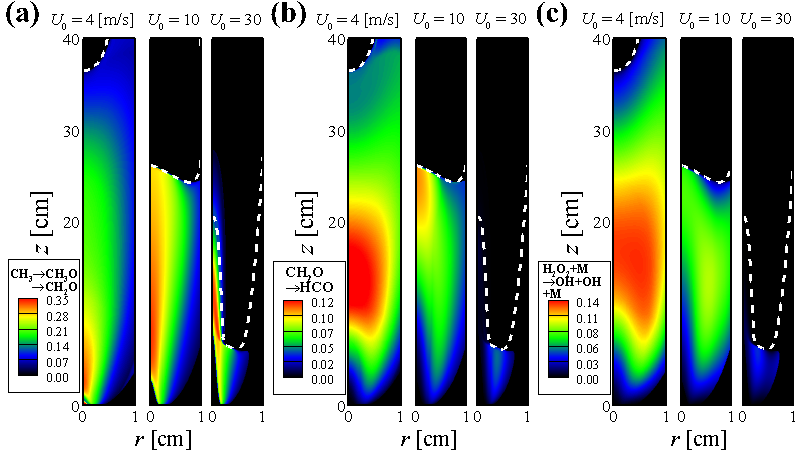
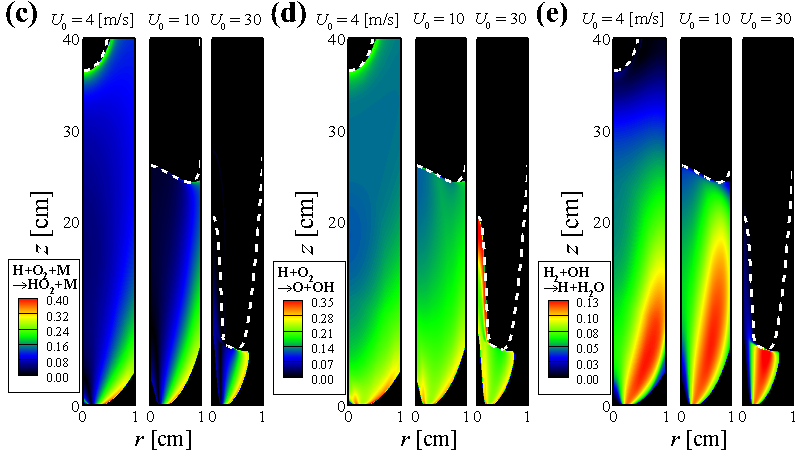
- From Chemical Explosive Mode Analysis (CEMA), important variables and reaction steps for the MILD combustion and tribrachial edge flame regimes are identified.
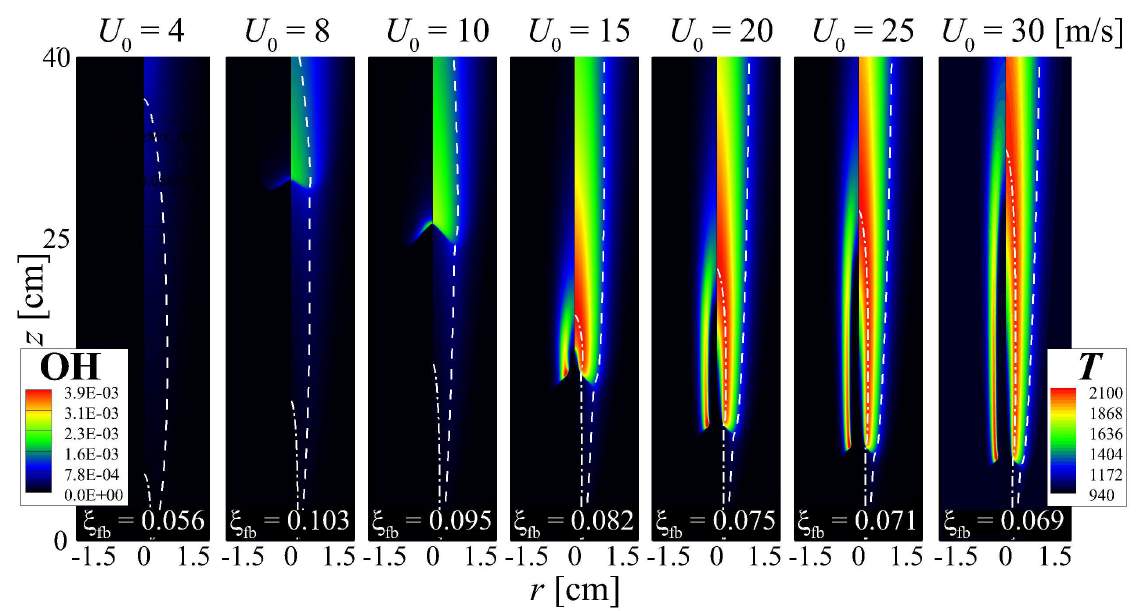
Isocontours of temperature and mass fraction of OH for autoignited methane/hydrogen jet flames
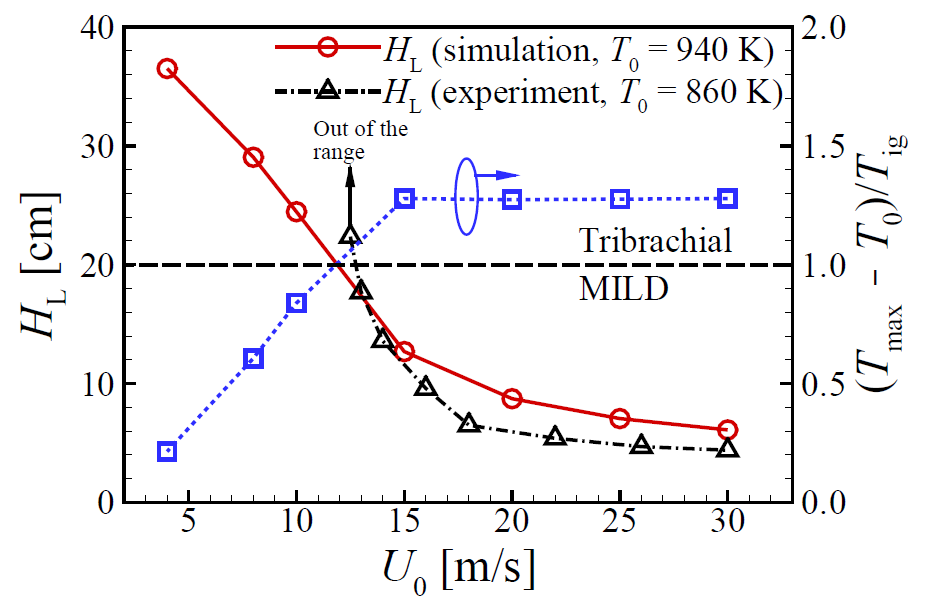
Liftoff and temperature variations with the increase of fuel jet velocity
Participation Index (PI) of important reactions for the MILD combustion (i.e., U = 4 m/s) and tribrachial edge flame regimes (i.e., U = 25 m/s)
Autoignited Dimethyl Ether (DME) jet flame
- to elucidate the U-shape liftoff height behavior with the variations of fuel jet velocity
- to elucidate the pyrolysis effect on the liftoff and flame structure
-
New Findings:
- DME is pyrolyzed by smaller species such as methane, hydrogen, and the pyrolysis effect increases as fuel jet velocity decreases
- Differential diffusion effect increases for the relatively-low jet velocity, which leads to the decreasing liftoff height behavior
Liftoff and temperature variations with the increase of fuel jet velocity
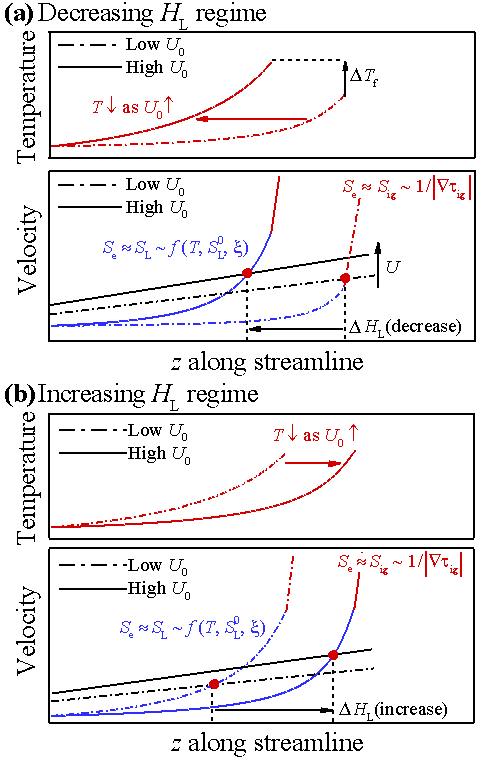
Schematic for the stabilization of autoignited laminar DME jet flames with (a) decreasing and (b) increasing liftoff regimes.
Flame Structures of DME Cool Flame
What is the cool flame?
- The phenomenon occurring as partial oxidation reactions of fuel are sustained at low temperatures ranging from 500 to 800K
- Preventing high local temperatures of the flame can block the causes of excessive soot and NOx emissions
-
New Findings:
- A new form of diffusion cool flame around the reaction zone where no temperature peak exists is discovered
- Various differences in ignition processes, heat release rates, and heat transfer phenomenon is analyzed.
Ignition delay time of n-heptane fuel according to the temperature and Low-temperature chemistry pathway of fuel oxidation
and self-sustaining diffusion cool flame of n-heptane fuel in the counter flow flame experimental setup
Heat release rate contour video for the Air temperature 600 K and 800 K
convection, diffusion, and reaction budgets along the centerline with temperature profiles